Normal Start: Becoming Sluggish
1. What was the starting Brix?
Solution: the most common cause of a sluggish fermentation that started normally is a lack of ethanol tolerance of the yeast conducting the fermentation. A quick calculation of the potential ethanol level from the starting Brix value should be done prior to selecting the yeast strain or conditions of fermentation. The theoretical maximum of ethanol that can be produced (if no carbon were directed to cellular metabolites and new cell growth) is 0.65 of the starting Brix. At theoretical maximum, a 24 Brix juice would yield 15.6% ethanol. The true theoretical maximum is rarely achieved because some sugar carbon must be expended on cellular components and growth. Also, some of the Brix value is due to extract (compounds other than sugar). A better conversion factor to use for potential ethanol is 0.6 times the Brix value. This will be a slight over-estimate as some ethanol will be lost during the fermentation but is a good value to use for planning purposes.
Once the potential ethanol at dryness is known, a strain easily able to attain that ethanol level should be used. The majority of yeast strains are tolerant to 11-12% ethanol. Commercial strains are tolerant to 14 to 18+% alcohol. In high Brix juices, the ethanol levels can easily be within this range and may exceed the tolerances of the strain selected. Tolerance is impacted by nutrient limitation, non-optimal fermentation temperatures, low pH and the presence of inhibitors. The tolerance level can be reduced 1 to 3% by the presence of these factors and multiple tolerance-impacting factors can synergistically reduce tolerance further. A temperature-shocked strain that is normally tolerant to 16% ethanol may only be tolerant to 12-13% under stressful conditions. The level of stress should also be evaluated in concert with the starting Brix level.
The Brix level at which the transition from the rapid onset rate of fermentation to the ethanol impacted rate can be an indicator of an impending issue with ethanol tolerance. Under tolerant conditions the transition will be around 11-12% ethanol, if it occurs at a much lower ethanol level, for example 8%, then a good rule of thumb is to assume the maximal tolerance level will be similarly reduced. If the strain was normally tolerant to 16% ethanol then its maximal tolerance under these conditions would be roughly 13%.
Stimulating these types of sluggish fermentations can be challenging. A first step would be to aerate to provide survival factors for enhanced ethanol tolerance. If the cells received some type of shock such as a temperature shock early on that impacts ethanol tolerance then the best course of action will be to remove the current biomass via settling and racking and to reinoculate with an adapted inoculant.
2. Were nutrients added?
Solution: Grape juice is often limiting in the nutrients needed to sustain fermentation rates and nitrogen is most often the limiting nutrient. Supplementation of the juice with nitrogen can help sustain fermentation rates. However it is important to not over-supplement or to supplement competitor populations. Yeast strains vary in nitrogen and other nutrient requirements so strains with more fastidious requirements should not be used if those requirements will not be met. Early and mid fermentation additions can be beneficial but nutrient addition after the ethanol tolerances of the strain have been exceeded may not have an impact. Also nutrient addition will not have an impact if some other factor (accumulation of an inhibitory substance) is the cause of the arrest. Although nitrogen is most often the limiting nutrient in grape juice, other factors such as phosphate or a micronutrient may be the component that is lacking. A juice chemical analysis should be performed to determine what components may be limiting growth and fermentation.
3. What strain was used?
Solution: nutrient requirements and ethanol tolerance varies by strains, even among commercial strains. With indigenous strains the requirements and tolerances may not be known. It is a misconception that the heartiest strains will be the ones that dominate a fermentation. There is no direct relationship between ability to dominate a fermentation and ability to tolerate ethanol. In fact, often the inverse is true. Strains with the highest tolerances to ethanol have those tolerances because they have higher basal levels of expression of stress-associated genes. Thus they are primed to respond quickly and favorably to any stressful situation that should arise. The presence of these stress-associated genes tends to slow growth rates and the fastest growing cells are those that have not expressed these stress-associated genes.
In mixed culture the strains not primed to resist stress will have the growth advantage. A strain with low ethanol tolerance may have dominated the fermentation in the beginning. In this case the sluggishness may be temporary as that population is surpassed by a more tolerant one. This generally requires that the initial strain die off in the population or settle from the fermentation and no longer be metabolically active, a process that happens naturally during indigenous flora fermentations. If the cells are still metabolically active they will count towards the maximal cell density and inhibit growth of the more hearty strain. The actual cell density sensors used by Saccharomyces during batch grape juice fermentation are unknown, but the presence of metabolically active but non-fermenting cells can inhibit the growth of cells that are both metabolically active and fermenting. In this case the yeast lees should be encouraged to settle, the wine racked off of the lees and reinoculated with an adapted inoculant.
4. What is the temperature?
Solution: ethanol tolerance is impacted by temperature. Tolerance is greatest at moderate temperature (20-25°C/68-77°F). Above or below this level ethanol will be inhibitory at a lower concentration. If the fermentation has gotten too cool, generally warming it will restore both ethanol tolerance and fermentation rates. Adaptation to high temperature requires different types of changes to the cell plasma membrane than does adaptation to ethanol. Thus the cells cannot simultaneously adapt to both and will try for a happy medium that winds up reducing both maximal temperature and ethanol tolerance. Fermentations cool naturally as they progress, and temperature issues generally only arise due to failure of a tank heating or cooling system or to errors in adjustment of tank temperatures. If a heat shock has occurred, then the wine should be racked off the yeast lees and reinoculated using an adapted inoculant.
5. Did temperature fluctuate?
Solution: temperature can be inhibitory even if it was not sustained at an inhibitory level. Transient temperature shocks can lead to reduced tolerances to ethanol that are not immediately apparent. Shocks during the early stages of fermentation that are corrected and seem to not have impacted fermentation rate can be manifest later on in fermentation as a decrease in innate ethanol tolerance. This is again because the transient shock to the population forced compositional changes in the plasma membrane that preclude developing a membrane that is highly ethanol tolerant. If a shock has occurred early on and there is the potential to exceed the ethanol tolerance of the inoculant, a new inoculant should be prepared and added. It may not be possible to rack off the existing biomass at this time so the new inoculant may have difficulty implanting but it could still be used. Under these conditions encapsulated yeast-in-bag preparations could be used to complete fermentation, as long as the conditions of fermentation did not exceed the tolerances of the encapsulated yeast. The encapsulated yeast do not need to grow and are primed to be tolerant to ethanol. Commercial restarter strains are also available that are very tolerant of both inhibitory conditions and biomass of other strains and can also be an option.
6. Are other microbes present?
Solution: if fermentation conditions allow the proliferation of other microbes, particularly of Lactobacillus, growth and end-product production of the bacteria can be inhibitory to the yeast and lead to an arrest of fermentation. Sluggishness arises either because the bacterial metabolites that are accumulating, such as acetic acid, are themselves inhibitory to yeast metabolism or because of impacts on yeast ethanol tolerance due to micronutrient or oxygen consumption and depletion. A temperature spike can lead to a bloom of lactobacilli if they are present in the fermentation. The accumulation of these populations is frequently inhibitory and addition of sulfite does not seem to be effective because the damage has already been done (depletion of nutrients or accumulation of inhibitors). In this case the wine should be separated from the biomass, a process that may require more than simple settling, and a new adapted inoculant should be used.
7. What was the juice microbial bioload?
Solution: if non-Saccharomyces organisms (wild yeasts or bacteria) were allowed to proliferate prior to or during the early stages of fermentation, fermentation onset may still appear to be normal. The inhibitory compounds produced by the other organisms may not become inhibitory until a certain ethanol concentration is reached. There are many synergistic reactions between ethanol and other inhibitory compounds. Likewise if the wild organisms have depleted the environment of survival factors such as fatty acids and sterols, the yeast may not show an impact until ethanol levels have become elevated. Early microbial activity can therefore show no immediate impact but lead to slower fermentation rates once ethanol has accumulated. As with bacterial blooms later in the fermentation these fermentations can be difficult to rejuvenate. If the cells have arrested growth addition of survival factors may postpone death but not revitalize fermentation. If the viability but not fermentative activity of an arrested culture is sustained then implantation with another strain may be difficult. The introduced strain will recognize the existing biomass density and not grow to exceed the maximal density. It is better to have had the cells die and not be contributors to cell density than to help sustain them in a fermentatively inactive state. The best solution may be encapsulated yeast as they are less sensitive to cell density impacts on metabolic activity.
8. Were any additions made?
Solution: disturbing an active fermentation can sometimes lead to sluggishness and arrest. Blending of fermentations that are at different stages and compositions may stress both cultures, for example blending a high pH high ethanol fermentation with a low pH low ethanol fermentation may shock the high pH ferment by the drop in pH and shock the low ethanol fermentation by the addition of ethanol. If the shocks are not too severe then the slow down in fermentation rate may be temporary. It is generally a good idea to hold off on adjustment of active fermentations until they are done.
Some fermentations have been arrested by sulfite addition, by addition of home-grown lactic acid bacterial starters, by use of inert gas to mix in a component (stripping of oxygen). The impact of these additions depends upon the ethanol concentration at which they are made, the yeast strain used, and the type of addition. Not all fermentations will become sluggish after an addition so it is important to understand the extenuating factors that will make fermentations more sensitive particularly the relationship between the ethanol level of the fermentation and the maximal tolerance of the fermentation.
9. What is the solids level?
Solution: juices that settle with a low solids level or if solids have been stripped from the juice due to heat treatments and subsequent processing prior to fermentation onset a sluggish fermentation can result. The figure shows the same juice with different solids levels. Following settling the solids level was moderately deficient (1.25%). The juice was further clarified via filtration to reduce the solids level to undetectable by gentle centrifugation. The collected solids were then used to boost the solids level of some of the juice by 5% (6.25%).
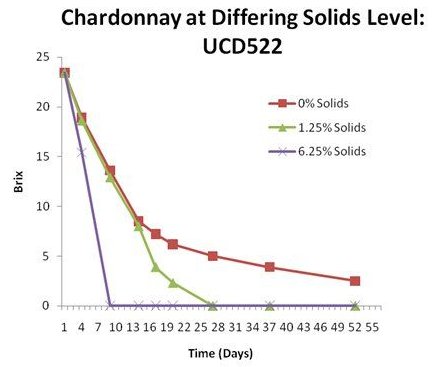
The impact of solids on fermentation rate is not well understood and several theories exist as to the reason solids benefit fermentation rate: entrapment of oxygen, stimulation of carbon dioxide bubble formation and release, provision of nutrients, provision of a solid surface for yeast adherence, removal of inhibitors via enzymatic degradation or entrapment, and facilitating mixing due to carbon dioxide evolution. The shapes of these curves offer some suggestions as to the impact of solids. Comparing the stripped solids (0%) to low solids (1.25%) fermentations the initial rates of fermentation are the same. The low solids condition shows a transition point at around 12% ethanol, which is typical for UCD522. This suggests that solids are impacting rate but not completion of the fermentation. The stripped solids condition shows a transition point at a much lower ethanol concentration, 9%, indicating that ethanol tolerance has been impacted. This suggests one role of solids may be to provide oxygen or survival factors needed to attain optimal ethanol tolerance of the culture. The high solids situation shows stimulation of fermentation rate as well as of ethanol tolerance. The curve is rapid and shows no transition point (fermentation completed at 14% ethanol). Thus the solids level can have a profound impact on fermentation rate, sluggishness and ethanol tolerance. Solids should be between 2 and 5%. If a low solids juice has arrested they can be difficult to restart. We have found that simple nutrient addition does not address the problem of low solids suggesting their role is not simply in the provision of macro or micronutrients. Dispersion of carbon dioxide may be important in the stimulation of fermentation rate and completion. Encapsulated yeast treatments may be effective in getting these fermentations to complete.
10. Was the fermentation aerated?
Solution: oxygen should be thought of as an important nutrient. Oxygen is required for the biosynthesis of sterols and unsaturated fatty acids, both of which are important components of ethanol-adapted membranes. Grape sterols and fatty acids exist in the medium but these are not always the optimal ones that the yeast needs. If starved for fatty acids the yeast will take up fatty acids from the medium and if there are any inhibitory bacterial fatty acids present those will be taken up by mistake. For these reasons it is a better strategy to provide the ferments with oxygen so that the cells can make their own fatty acids. There is a lot of competition for juice oxygen, other microbes, polyphenol and other oxidase activity, chemical reactants, so it is important to make sure the yeast will benefit from the addition. If a fermentation starts to slow, the first treatment would be to expose to oxygen as this can allow the cells to adjust membrane composition. If delayed too long then the addition may not be effective for the entire population.
