The second type of problem that can arise during fermentation is the development of an unpleasant aroma. What is deemed "objectionable" may depend upon the circumstances and aromatic profile of the wine. Some compounds, such as hydrogen sulfide (H2S), are universally perceived as negative while others, like the floral esters, may be desired in some wines or at some concentrations. Yeast strains vary in their abilities to produce aromatic substances and the production of these compounds is influenced by the composition of the grape juice. It can be difficult to predict the fermentation aroma outcome from the starting juice chemistry and yeast strain. At low levels, some compounds that are offensive at high concentrations, such as dimethyl sulfide, may exert a positive influence on the wines. However, obtaining a precise level of production of these compounds is problematic.
Yeast are also responsible for the liberation of some grape aroma characteristics considered to be positive. Yeast hydrolase activity may release aromatic components from bound precursor forms in the juice or yeast activity may modify grape components. Generally such activities are desired but there are circumstances, such as the oxidation of grape C6 and C8 alcohols to their corresponding aldehydes, that may yield too strong of an aldehyde (green, grassy, earthy, mushroom) signature to the wine.
Types of Fermentation-Driven Off-Characters
The types of off-characters that can form during fermentation fall generally into one of three main categories: Sulfur-containing volatiles, esters, and fusel compounds. Aldehydes, volatile acids and free aromatic fatty acids can also be formed under some conditions and may negatively impact the aroma profile of the wine. Hydrogen sulfide is typically generated from the reduction of sulfate during amino acid biosynthesis. Esters and fusel compounds also come from amino acid biosynthesis or degradation. The more complex S-volatiles can be generated via degradation of the S-containing amino acids, cysteine or methionine, or their metabolic derivatives within the cell. S-containing volatiles can also come from degradation of S-containing vitamins, from S-containing plant metabolites or from S-containing pesticide/fungicide residues. In the vast majority of cases, the role of pesticide/fungicide residues can be ignored as it is rare that these components would have been used close to harvest.
Off-character formation may occur prior to the establishment of Saccharomyces, during the early stages of fermentation, during the active phase of fermentation, at the end of fermentation or during aging on the yeast lees. Pre-fermentation aromas often come from the activity of non-Saccharomyces yeasts and bacteria. The most common early off-characters are acetic acid (a pungent note) and ethyl acetate (glue, solvent).
During active biomass accumulation, H2S and ester production may occur. In this case, ester formation is stimulated by the presence of nitrogen, indicating that biosynthetic reactions are the source of these compounds. Once active growth has diminished and ethanol is accumulating, amino acid degradation occurs and, at this time, additional esters and fusel compounds may be produced. The fusel compounds come from the degradation of amino acids as nitrogen sources via the Ehrlich pathway.
As fermentation ends, the cells enter a resting phase. At this time, there is a release of accumulated intermediates to the environment. The cells are entering a non-growth phase and are filling cellular storage pools to capacity. Excess nutrients or components that would be energetically costly to convert to storage forms are excreted. Some components may be degraded in the conversion process to storage. Fatty acid esters may form at this time, as well as some complex S-volatiles. This stage is characterized by a release of amino acids and other components to the environment and the availability of these components is stimulatory to the growth of the ML bacteria. Free fatty acids may also be released at this time. Generally, aromatic fatty acids are not produced at levels above their thresholds of detection. At low levels these compounds can add to complexity, but at higher levels they can impart a rancid or cheese note to the wine. These characters can also be produced by bacterial activity, so the role played by Saccharomyces in these types of defects is unclear.
Aldehydes derived from sugar and carbon catabolism, such as acetaldehyde (apple, bruised apple and nutty; depending upon the matrix and concentration), can be found in wine but generally not released in high enough levels during fermentation to be problematic. Grape alcohols, such as the C6 hexanols can be oxidized to aldehydes by yeast activity. The C6 aldehydes can contribute stronger green or unripe characters to the juice and wine than their progenitor alcohols. Oxidation of the C8 alcohols to aldehydes leads to mushroom and earthy characters, described by some as a charred wood note.
If the wine remains in contact with the yeast lees, additional aroma compounds may form. Yeast cell autolysis, which is the breakdown of internal cellular structures and the release of hydrolytic enzymes, can occur. Fatty acids and other compounds may be released that impact mouth feel in addition to impacts on aroma. S-volatile formation may also occur, depending upon the composition of the yeast and the chemical conditions of the wine.
Classes of Fermentation Off-Characters:
The S-Containing Volatiles:
Sulfur-containing volatile compounds are perhaps the most challenging of the off-aromas that can form during fermentation. These compounds are reactive and can exist in different chemical states, making both detection and removal difficult. At some points, the off-aroma may seem to have disappeared, only to return later in processing, as chemical conditions change. More oxidized forms may be non-volatile or non-aromatic, but if the environment becomes more reduced, they can be restored to the aromatic reduced form and reappear as a sensory defect.
Hydrogen sulfide: The formation of volatile sulfur compounds in wine is a chronic problem that occurs globally. Many factors have been associated with the appearance of hydrogen sulfide (H2S) in wine and it has been difficult to design fermentation management strategies that guarantee the absence of sulfide during production. This is, in large part, due to the fact that there is a significant influence of strain genetic background on both the level of sulfide produced and the conditions under which it appears. There is also a strong interactive impact of juice composition that may exacerbate the underlying genetic differences across strains. Hydrogen sulfide can be produced in yeast as a consequence of the reduction of sulfate that is needed in order to synthesize the sulfur containing amino acids, methionine and cysteine. In this case, it has been hypothesized that more sulfide is reduced than can be incorporated, such that the excess sulfide is released from the cell as hydrogen sulfide.
However, hydrogen sulfide has recently been shown to perform an important signaling function. H2S will arrest the respiration phase and signal the onset of fermentation. This allows the population of cells to coordinate metabolic activity. H2S is deliberately made and released to coordinate rapid fermentation onset. This serves to accelerate strain control of an environment by rapid generation of ethanol by the population, and signals the availability of sugar to the culture. In this case, H2S formation would not be responsive to nitrogen availability as it is not being released under conditions of starvation. Bacterial respiration will likewise be inhibited by the presence of H2S and this metabolite may play an underappreciated role in allowing ecosystem dominance by Saccharomyces. In this case, there would be a strong selective advantage to the production of H2S under certain environmental conditions. In contrast, it is difficult to imagine the benefit of being metabolically sloppy and reducing more sulfate than can be consumed by cellular metabolism. The strain variation in H2S formation and high level production found particularly in vineyard isolates, indicates that formation of H2S is beneficial to the strains.
H2S can also be formed from the degradation of sulfur-containing amino acids. If the levels of methionine and cysteine are high relative to other nitrogen-containing compounds, they can be used as nitrogen sources. In this case, the nitrogen is what is of value to the cell, not the sulfur. The sulfide can then be released either as H2S or as a thiol or some other aromatic waste form of organic sulfur. Some rootstocks have been shown to yield fruit with relatively high concentrations of the sulfur-containing amino acids. Inorganic sulfur used as an antifungal treatment on the grapes can also be reduced to H2S during fermentation. This process requires the reducing conditions established by yeast metabolism, but it is basically a chemical reduction that is thought, for the most part, to be strain independent.
Yeast strains can produce sulfide early in the fermentation, typically as cell biomass is expanding and fermentation rates are at their highest, or it can be produced late in fermentation, as the cells remain in contact with the wine. This latter formation of hydrogen sulfide is thought to be due to the degradation of sulfur-containing amino acids, and does show a genetic influence as some strains appear to have more of a tendency to produce sulfides under these conditions than others.
During yeast-lees aging, it is also thought that H2S can be produced directly from SO2. If sulfite reductase is active and there is a source of electrons to generate reduced cofactors, the sulfite can be reduced to sulfide and released.
Higher Sulfides: More complex sulfides can also be formed in wine. The commonly found sulfides are shown in the following table.
Volatile Sulfides Found in Wine
|
Compound |
Aroma Description |
Source |
Concentration in Wine (µg/L) |
Putative Thresholda(µg/L) |
|
Hydrogen sulfide |
Rotten eggs, fecal |
Sulfate reduction, S-amino acids |
nd-370 |
50-80 |
|
Dimethyl sulfide |
Cooked corn, cooked asparagus, cabbage, molasses, canned vegetable, skunk, clam |
S- amino acids |
ndb-480 |
10/25/60 |
|
Diethyl sulfide |
Garlic, onion, strong garlic |
S- amino acids |
nd-10 |
0.9 |
|
Dimethyl disulfide |
Cooked vegetable, strong onion Cabbage |
S- amino acids |
nd-22 |
29 |
|
Diethyl disulfide |
Strong onion, burnt rubber |
S- amino acids |
nd-80 |
4.3 |
|
Dimethyl trisulfide |
Onion, garlic, meat, green, cabbage |
S- amino acids |
nd- 0.25 |
0.2 |
1Thresholds vary by the matrix of the solution tested. The variation in number indicates different matrix conditions were evaluated. Thresholds should always be viewed with caution.
2 nd= not detected
The higher sulfide compounds are believed to largely generate from the degradation of the S-containing amino acids. Spiking wines with methionine, cysteine or the cysteine-containing tripeptide glutathione leads to the formation of these compounds in juices and wines. Some of these compounds appear to continue to increase during storage of the wine after yeast activity has ceased, suggesting that there are precursor forms present in wine that, as the reductive conditions of the wine change, generate S-volatiles. In beer, for example, dimethyl sulfide can form from reduction of Dimethyl sulfoxide derived from S-methylmethionine. This pathway has not been shown to exist in wine, where dimethyl sulfide is believed to come from the degradation of cysteine, glutathione, methionine or S-adenosyl–L-methionine. Some higher sulfides may also come from degradation of S-containing pesticides, but this is a rare occurrence and more often these characters are derived from catabolism of S-amino acids and their derivatives, glutathione and S-adenosyl-L-methionine.
Thiols and Thioalcohols: Mercaptans and thioalcohols can also be found in wine. These components likewise are believed to derive from degradation products of S-containing amino acids and their derivatives or from the interaction of H2S with acetaldehyde, which forms the reactive 1,1-ethanedithiol, and other reactive components in wine. Some of these products are reactive themselves, leading to even more diverse S-volatiles. The chemical reactivity of these compounds in combination with a host of potential reactants in wine and very low thresholds of detection, has made it challenging to delineate the true pathways by which they are formed.
Thiols and Thioalcohols Found in Wine
|
Compound |
Aroma Description |
Source |
Concentration in Wine (µg/L) |
Putative Thresholda(µg/L) |
|
Methanethiol (Methyl mercaptan) |
Cooked cabbage, rotten eggs |
S- amino acids |
ndb-16 |
0.2/2/12 |
|
Ethanethiol (Ethyl mercaptan) |
Onion, rubber, natural gas, fecal |
S- amino acids |
nd-12 |
1.1 |
|
2-Mercaptoethanol |
Poultry, barnyard, solvent |
S- amino acids |
nd-180 |
1000 |
|
3-(Methylthio)-1-ethanol |
Green beans |
S- amino acids |
nd-70 |
|
|
Methionol (3-(methylthio)-1-propanol) |
Potato, cauliflower |
S- amino acids |
nd- 6300 |
10-50 |
|
4-(Methylthio)-1-butanol |
Onion, garlic, earthy |
S-amino acids |
nd-180 |
100 |
|
2-Methyl-3-furanthiol |
Cooked meat |
Thiamine degradation |
nd-0.300 |
|
|
Furfurylthiol |
Roasted coffee, meat, popcorn |
Toasted barrel wood extractives and H2S |
nd-0.050 |
0.0004 |
|
Benzylthiol |
Smoky, flintstone |
H2S; Formed during aging |
nd-0.015 |
0.0003 |
1Thresholds vary by the matrix of the solution tested. The variation in number indicates different matrix conditions were evaluated. Thresholds should always be viewed with caution.
2 nd= not detected
The mercaptans can form disulfides in wine that can be reduced back to the mercaptan by sulfite or ascorbic acid treatment. The disulfide form does not react with copper, and if mercaptans are present in the wine, it may be necessary to treat the wine with ascorbate prior to addition of copper to remove the mercaptans along with H2S.
Mercaptoethanol is generally not found in wines above an odor threshold. However,,,, this compound is highly reactive and is formed in high levels in synthetic fermentation conditions. Given its reactivity, it is not surprising that it is seldom detected, as such, in wines.
Methionol is the end product of the deamination of methionine via the Ehrlich pathway. Methionol comes from the α-keto acid 3-(methylthio)-propanoic acid. The acid can be decaboxylated and then reduced to an alcohol. In some wines, methionol is the major S-containing volatile compound. Detection of its tuber note may be masked by other grape components. 4-methylthio-1-butanol and 2-mercaptoethanol can be formed similarly from homocysteine and cysteine via the Ehrlich pathway.
2-Methyl-3-furanthiol and its disulfide, bis(2-methyl-3-furyl)disulfide are thought to form from the degradation of thiamine. Other thiol compounds arise during barrel aging and seem to be present in higher concentrations if H2S is also present, but the exact mechanisms of synthesis are unknown.
Thioacetates and Thiazoles: Other forms of S-volatiles can also be found in wine. These compounds are derived directly, or indirectly, from S-containing amino acids or from thiamine.
Thioacetates and Thiazoles Found in Wine
|
Compound |
Aroma Description |
Source |
Concentration in Wine (µg/L) |
Putative Thresholda(µg/L) |
|
Methyl thioacetate |
Cheese, rotten vegetables, sulfurous |
Methionine, methanethiol + acetylCoA |
ndb-20 |
300 (beer) |
|
Ethyl thioacetate |
Cheese, onion, meaty, coffee, burnt, sulfurous |
Ethanethiol + acetylCoA |
nd-56 |
40 |
|
Benzothiazole |
Rubber |
Thermal degradation of cysteine, thiamine |
nd-14 |
50/200 |
|
2-Methyltetrahydro-thiophene-3-one |
Metallic, butane |
Thermal degradation of cysteine, methionine, thiamine |
nd-167 |
1Thresholds vary by the matrix of the solution tested. The variation in number indicates different matrix conditions were evaluated. Thresholds should always be viewed with caution.
2 nd= not detected
Methyl and ethyl thioacetate appear to derive from the reaction of methanethiol and ethanethiol, respectively, with acetyl-CoA. The reaction appears to be catalyzed by alcohol acetyltransferases, the same enzymes used for the generation of the family of acetate esters. This acetylation may be a detoxification mechanism for the thiols. High thiol formation has been correlated with release of high levels of H2S by some strains, so the appearance of these compounds may indicate conditions of breakdown of S-containing amino acid pools. Their production is also strain dependent. The thioacetates have much higher thresholds of detection than their thiol precursors. Generally, the thioacetates are not over their thresholds of detection in most wines. However, like all esters, they will hydrolyze over time during wine aging, leading to the generation of the original thiol and acetate. These compounds can serve as a reservoir of off-characters in wine.
The azoles, benzothiazole and 2-methyltetrahydro-thiophene-3-one, are formed during the thermal degradation of S-containing amino acids and thiamine in the presence of carbonyls. These compounds have also been found in grapes. Thus, they can form from yeast metabolites in heated wines or juices. Benzothiazole is generally found below its threshold of detection with the exception of "mousy" wines, where it can contribute to the off-odor of the wines. The mechanism of formation under these conditions is not known but it may be accelerated due to the production of carbonyls by the lactic acid bacteria. 2-Methyltetrahydro-thiophene-3-one has been found following heating of model solutions containing either methionine, cysteine or thiamine in the presence of dicarbonyls. The exact mechanism of formation in wine is unknown.
Sulfur compound chemistry is complex. Several studies have tried to correlate appearance of these compounds with the formation of H2S. Direct sulfylation by H2S of precursor compounds has not been demonstrated in wine or juice conditions. The appearance of H2S simultaneously with compounds that are degradation products of the S-containing amino acids may simply indicate that degradation is occurring and H2S is being made along with the other derivatives.
Yeast can also degrade S-containing cysteinylated compounds found in grapes, forming thiols that are important to the character of several varietals. These compounds are not considered to be off-characters in wine, although, if present at very high concentrations, they may be. Typically these compounds are desired at the levels found in wines.
Sulfur compounds used as antifungal agents in the vineyard can lead to the formation of S-off-characters in wine. In many regions these compounds are no longer used and more effective and targeted treatments are available. However, if an odd off-odor develops in a wine, the vineyard should be evaluated as a potential source of the problem.
The Esters
Esters can contribute positively to the aroma of a wine. In low concentrations, these compounds are perceived as generically fruity or floral and can boost the awareness of the innate fruit and floral characteristics of the grape varietal. However, if they are present in too high a concentration, they can mask the other varietal aromas and yield a synthetic fruit or fruit candy aroma to the wine, decreasing wine complexity. As with some of the sulfur compounds, low concentrations are desirable, high concentrations may negatively impact the wine. The level of ester production that will cause a problem is dependent upon the composition of the individual wine matrix and its characteristic varietal odor-impact compounds.
There are two classes of esters formed in wine: the ethyl esters and the acetate esters. Esters derive from the combination of an alcohol with an acid. Enzymes transfer the acyl moiety (acid group) of an acyl-CoA conjugate to a receptor alcohol. The alcohol can be ethanol or any alcohol produced as a degradation product by yeast cells. Aside from ethanol, common alcohols found in esters derive from the degradation of amino acids. The most common acyl-CoA molecule found in yeast is acetyl-CoA and, thus, the most common esters are acetate esters. At low concentrations, esters can add complexity to a wine and, due to their generic floral and fruity notes, enhance the aroma characteristics of the varietal. High concentrations of esters can be objectionable, depending upon the matrix of the wine. Wines containing more than 90 mg/L of ethyl acetate or 200 mg/L of total esters are considered defective.
Esters are formed for three key reasons. First esters are generally less toxic than their alcohol or acidic precursor molecules, so the formation of esters can be a detoxification mechanism. Second, esters are insect attractants and signaling molecules. The formation of esters serves to attract insects to the fermentation and culture of yeast so that they may be spread. A third reason for forming esters is the regeneration of free Coenzyme A from its conjugates. During fatty acid biosynthesis and degradation fatty acyl-CoA, molecules are formed. If biosynthesis or degradation is interrupted, the bound Co-A can be released by reaction with an alcohol.
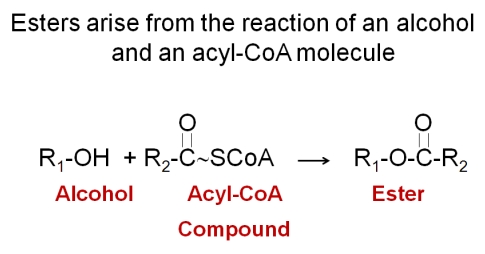
In addition to synthesizing esters, yeast also make esterases that cleave these compounds to their original alcohol and acid moieties. Ester production varies with yeast strains and there is a family of enzymes responsible for ester formation and degradation. Differences in the expression or activity of this class of compounds can account of the variation in ester levels in different yeast strains. The common esters found in wine are shown in the following table.
Common Esters Found in Wine
|
Compound |
Aroma Description |
Source |
Concentration in Wine (µg/L) |
Putative Thresholda(µg/L) |
|
Ethyl acetate |
Glue, solvent, nail polish, vinegar |
Cell carbon metabolism |
2-150 |
7.5 |
|
Ethyl butanoate |
Floral, fruity |
Amino acid and fatty acid degradation |
70-2200 |
20 |
|
Ethyl hexanoate |
Green apple, unripe fruit |
Fatty acid degradation, grape acids |
150-2800 |
5/14 |
|
Ethyl octanoate |
Soapy, floral |
Fatty acid degradation, grape acids |
130-2700 |
2,5 |
|
Ethyl decanoate |
Soapy, floral |
Fatty acid degradation |
14-850 |
200 |
|
Ethyl propanoate |
Fruity |
Amino acid degradation |
1800 |
|
|
Ethyl-2-methyl propanoate (ethyl isobutyrate) |
Fruity, pineapple |
Amino acid degradation |
30-480 |
15 |
|
Ethyl-2-methyl butanoate |
Fruity |
Amino acid degradation |
1-30 |
1/18 |
|
Ethyl 3-methyl butanoate (ethyl isovalerate) |
Fruity, berry, blackberry |
Amino acid degradation |
2-36 |
3 |
|
Ethyl lactate |
Strawberry, fruity |
Cell carbon metabolism |
0.2-390 |
150 |
|
Isoamyl acetate |
Banana, tropical fruit |
Amino acid degradation |
115-7400 |
30 |
|
2-Phenethyl acetate |
Rose, flowery, honey |
Amino acid degradation |
0.5-750 |
250 |
|
Isobutyl acetate |
Banana, tropical fruit |
Amino acid degradation |
nd*-1600 |
1600 |
|
Hexyl acetate |
Fruity, perfume |
Grape C6 alcohols |
nd-4800 |
700 |
|
2-Methyl propyl acetate |
Banana, tropical fruit |
Amino acid degradation |
1600 |
|
|
2-Methyl butyl acetate |
Banana, tropical fruit |
Amino acid degradation |
160 |
|
|
3-Methyl butyl acetate |
Banana, tropical fruit |
Amino acid degradation |
30 |
1Thresholds vary by the matrix of the solution tested. The variation in number indicates different matrix conditions were evaluated. Thresholds should always be viewed with caution.
2 nd= not detected
Ethyl Acetate:
Ethyl acetate is the principle aroma component of wine vinegar. It is described as glue, solvent, nail polish in high concentrations. Ethyl acetate is produced by the wild yeasts, particularly Hanseniaspora uvarum (Kloeckera apiculata). H. uvarum is a normal resident of the berry surface, and is often found in high numbers in juice. It is readily inhibited by inoculation with Saccharomyces, but is more tolerant of low temperature conditions than Saccharomyces. Pre-fermentation cold soaks encourage a bloom of H. uvarum. Since ethyl acetate is an ester, it is volatile and may be driven off during fermentation if fermentation is vigorous enough. It may also be hydrolyzed at the low pH of wine. However, if a high concentration is present, the compound can still be present at the time of bottling in a high enough level to be detected.
Fatty acid ethyl esters:
The C10 to C12 fatty acid esters derive from either the degradation of fatty acids or from fatty acid biosynthesis. In both processes, Coenzyme A is involved. In biosynthesis, acetyl molecules are added sequentially to the growing acyl chain to yield C16 and C18 fatty acids. If biosynthesis is arrested, the cell will need to free the CoA from the conjugate, but at the same time not release potentially toxic fatty acids. The formation of an ester between the fatty acid moiety and ethanol serves to free the CoA as well as to create a non-toxic volatile ester that can be secreted from the cell. Similarly, fatty acid degradation occurs by attaching the fatty acid to a Coenzyme A molecule, then degrading the molecule in two carbon acetate units. As with biosynthesis, if degradation is interrupted, CoA will be released via the formation of an ester of the attached acyl chain. Fatty acid ester formation occurs when oxygen is present then consumed and no longer available. Oxygen is needed for synthesis of unsaturated fatty acids. Fatty acid ester formation can also occur late in fermentation as cellular fatty acids are being degraded.
The C6 and C8 esters could arise from fatty acid catabolism or biosynthesis as well. However, the grape makes C6 and C8 alcohols and the esters of C6 and C8 compounds may represent detoxification of these components if they are taken up by the cells.
Esters from amino acid degradation:
Both ethyl and acetate esters can form from the degradation or biosynthesis of amino acids. Biosynthetic and degradative reactions generate acid and alcohol forms of the carbon skeletons of amino acids. The acidic forms can react with ethanol to form ethyl esters and the alcohol derivatives can react with acetylCo-A to form acetate esters. The most important esters from a sensory perspective come from the leucine family of amino acids and from phenylalanine. Esters are also formed from carbon skeletons of other amino acids, but these compounds are rarely near, let alone over, their thresholds of detection.
Since esters can form either during the amino acid biosynthesis or degradation process, it is difficult to assess the impact of juice nitrogen content and supplementation on ester production. Some studies have shown the highest ester content occurs with diammonium phosphate (DAP) supplementation (biosynthesis driven) while others have shown the opposite: that the highest ester content is amino acid content driven and inhibited by the use of DAP (degradation driven). Both conclusions are likely correct: it depends upon whether or not ester formation accompanies biosynthesis or degradation in the strain and juice conditions being evaluated.
Odor Impact Amino Acid Derivatives Found in Wine:
Another class of off-characters found as a consequence of yeast metabolic activity are the fusel compounds, alcohols, acids and aldehydes, derived from the degradation of amino acids via the Ehrlich pathway. These compounds can also be formed from the intermediates of amino acid biosynthesis. They are often precursors to the ester formation, but can have direct impacts on the aroma of the wine. The foul nature of high concentrations of some of the alcohol forms led to the characterization of these compounds as fusel oils. The alcohol forms are definitely in the chemical taint family of compounds, but the acids and aldehydes can also be responsible for off-notes in wine. In general, these compounds derive from the degradation of amino acids via the Ehrlich pathway or can come from biosynthetic intermediates formed during amino acid biosynthesis.
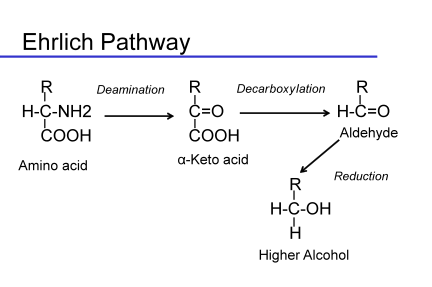
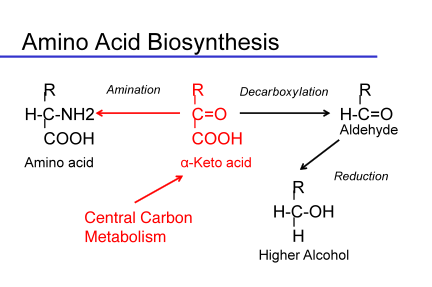
Like esters, fusel compounds may have positive effects at low concentrations but are negative at higher concentrations, above their thresholds. The common fusel compounds are shown in the following table.
Amino Acid Derivatives Found in Wine
|
Amino Acid Precursor |
Acid |
Aldehyde |
Alcohol |
|
Branched Chain Amino Acids Leucine Isoleucine Valine |
3-Methylbutanoate 2-Methylbutanoate 2-Methylpropanoate |
3-Methylbutanal 2-Methylbutanal 2-Methylpropanal |
3-Methylbutanol (isoamyl alcohol) 2-Methylbutanol (d-amyl alcohol) 2-Methylpropanol (isobutanol) |
|
Aromatic Amino Acids Phenyalanine Tyrosine Tryptophan |
2-Phenylacetic acid 3-Indoleacetic acid Hydroxyphenylacetic acid |
2-Phenylacetaldehyde 3-Indoleacetaldehyde Hydroxyphenyl-acetaldehyde |
Phenylethanol Tyrosol Tryptophol |
|
S-Containing Amino Acids Methionine |
3-Methylthiopropanoate |
Methional |
Methionol |
|
Other Amino Acids Threonine |
Propionate |
1-Propanal |
1-Propanol |
The aroma descriptors of these compounds that are found in wine, their concentrations and putative threshold levels are given in the table below:
Descriptors and Concentrations of Amino Acid Derivatives Found in Wine
|
Compound |
Aroma Description |
Concentration in Wine (µg/L) |
Putative Thresholda (µg/L) |
|
3-Methylbutanoate |
Bleu cheese |
3000 |
|
|
2-Methylbutanoate |
Cheese, sweaty |
3000 |
|
|
2-Methylpropanoate |
Cheese, rancid |
200000000 |
|
|
2-Phenylacetic acid |
Pollen, flowery, roses |
||
|
3-Methylthiopropanoate |
Chocolate, roasted |
||
|
Propionate |
Vinegar |
8100 |
|
|
2-Phenylacetaldehyde |
Honey |
||
|
Methional |
Cereal, potato, soup-like |
||
|
3-Methylbutanol (isoamyl alcohol) |
Nail polish, solvent |
30000 |
|
|
2-Methylbutanol (d-amyl alcohol) |
Nail polish, solvent |
65000 |
|
|
2-Methylpropanol (isobutanol) |
Solvent, chemical |
9000-175000 |
40000 |
|
Phenylethanol |
Floral, rose |
4000-200000 |
10000 |
|
Methionol |
Cabbage, potato, cauliflower |
140-5000 |
500 |
|
1-Propanol |
Solvent, chemical |
9-68000 |
500000 |
1Thresholds vary by the matrix of the solution tested. The variation in number indicates different matrix conditions were evaluated. Thresholds should always be viewed with caution.
- nd= not detected
In general, the alcohols are the major species present in wine. Aldehydes can be found in wine under aging conditions, if re-oxidation of the alcohols occurs. The aldehydes are as objectionable as their corresponding alcohols. The acid forms can also be found in wine. The aldehyde is the most toxic intermediate of keto acid degradation. The cell will either reduce the aldehyde to an alcohol or oxidize it to an acid as these forms are both less toxic than the aldehyde. Which form is made depends upon the availability of reduced cofactors in the cell. During fermentation, reduced cofactors are generally plentiful, so alcohols are made. But under some conditions, the acids will appear. Esters of the fusel acids and fusel alcohols can be found in wine. In other yeast species, it is thought that the fusel compounds and their esters are population signaling molecules. This may be true in Saccharomyces as well.
If the total fusel alcohol level is below 300 mg/L, the wine is described as fruity and pleasant containing peach and apricot aromas. Above 400 mg/L, the wine becomes pungent with a strong foul chemical taste and aroma. In wine, the total produced varies within this range, from less than 100 to greater than 500 mg/L. The individual compounds vary from 10-140 mg/L. The amounts formed show a strong stain dependence.
Aldehydes:
Since the formation of an SO2- aldehyde adjunct is one way cells become tolerant to sulfite, excessive sulfite use may lead to release of high concentrations of acetaldehyde that would not then be reconsumed by the yeast. But in general, when acetaldehyde is found in wine it comes from the chemical re-oxidation of ethanol during aging and oxygen exposure of the wine. Acetaldehyde levels in wine range from 1 – 160 mg/L. It has a putative threshold of 100 mg/L. It is described most often as sherry-like in wines, but has bruised apple and nutty notes as well. The higher aldehydes derived from amino acids can have strong nutty and rancid nutty notes at high concentrations and are made under the same chemical conditions conducive to acetaldehyde formation. At lower concentrations, they may confer notes of coffee, chocolate or stone fruits.
Prevention of Off-Character Production
Prevention of the formation of off-characters during fermentation can be tricky because production of these compounds varies dramatically by yeast strain. If the behavior of the yeast strain is known, nutrient management can be used to alter the pattern of formation of these compounds. Nutrients high in S-containing volatiles should not be used. In general, such nutrients are not commonly marketed for the wine industry; but if nutrients designed for a different commodity are purchased and used, the S-amino acid content may be too high.
Volatile compounds produced during fermentation may be driven off by the carbon dioxide stream. Whether this occurs or not will be dependent upon the vigor of the fermentation post-formation of the aroma compounds, the temperature, and the volatility of the compound. Lower temperatures favor retention and higher temperatures favor loss. If wine sitting on the yeast lees starts to show evidence of the formation of these compounds, the wine should be immediately racked off of the lees. With respect to ethyl acetate formation, the amounts made by Saccharomyces are inconsequential but the amounts formed by the acetic acid bacteria and Hanseniaspora uvarum can be so high that these compounds will not be lost from the wine during fermentation or subsequent processing. If the odor of ethyl acetate is noticed during a cold soak or other pre-fermentation treatment, that treatment should be halted and the fermentation inoculated immediately. If a juice or fruit from a particular vineyard is prone to off-character formation then a strain not producing those characters should be selected.
It is also important to note that when trying to select for formation of these compounds, they can be derived from biosynthetic or degradation reactions. These will occur at different times and under different conditions and, again, there will be considerable strain variation. It may be difficult to limit the formation of these compounds to the window that represents their positive formation.
If an off-character is found, particularly after active fermentation has ended, it may appear in the finished wine at the time of bottling. Some off-characters that may have appeared to have dissipated may return in the wine during aging or post-bottling. Thus, effective mitigation practices need to be employed at the winery.
Esters: Esters are volatile and will hydrolyze under wine making conditions, depending upon the level formed, temperature of the wine and conditions of storage. For the Saccharomyces fermentation esters under barrel aging conditions, they have mostly hydrolyzed in six months. Saccharomyces ester taints are rarely a problem in red wine production. Esters may be problematic in white wines due to the lower temperature of fermentation and aging of these wines. Less of the ester will be lost due to volatilization in a cooler environment and less will be lost due to hydrolysis. The grape solids level during fermentation will also impact ester retention, as the solids contain esterases that catalyze the hydrolysis of esters. The problem with reduction of unwanted esters is not the efficacy of treatments, but their impact on other aroma characters. Grape esters and other volatile compounds important to wine aroma may be lost in the efforts to eliminate esters. Some success has been reported with respect to using solids as a source of esterases, but these enzymes will not discriminate between desired and undesired esters. Yeast lees also contain esterases and incubation on the yeast lees will lead to the loss of wine esters, but such treatments are not neutral: other yeast-derived components will be synthesized at the same time that may or may not be desired.
Volatiles-stripping technologies can be used to remove esters from wine, but again, these techniques are not highly discriminatory. However, given the high thresholds of detection of esters, reduction of the ester content in a fraction of the wine and blending back may be sufficient to reduce the ester content to a desired level.
Ethyl acetate can be problematic as it can be formed in the wine post-fermentation by the action of the acetic acid bacteria. Under these conditions, active fermentation has ceased and volatile compounds will not be driven off of the fermentation. Ethyl acetate can also be a problem if formed pre-fermentation by Hanseniaspora. Hanseniaspora can produce very high concentrations of ethyl acetate that do not dissipate during typical fermentation and storage conditions, simply because too much of the compound is present and it has a relatively low threshold of detection. In this case, volatiles stripping may be the only option to salvage the wine.
Sulfur compounds: Sulfur compounds are also volatile and can be removed by fermentation gasses if formed early enough in the fermentation. H2S produced transiently during cell growth and the early stages of fermentation is frequently dissipated by carbon dioxide during the fermentation. However this does not always happen. Again, whether the H2S is lost or not depends upon the temperature, vigor of the fermentation and total amount of the sulfide made. If the H2S or S-volatile is made after the most vigorous phase of fermentation, it will not be dissipated. Winemakers will then have to use splashing or increased exposure of the wine surface to air to allow enhanced volatilization of the S-volatiles. This works as long as the presence of air does not lead to the formation of less volatile neutral derivatives of the S-compound. The oxidized versions may be retained in the wine and when the wine becomes chemically re-reduced during aging, the S-compounds can be reduced back to their aromatic form. Using an inert gas like carbon dioxide, nitrogen or conducting the volatile stripping process under a modified atmosphere, should limit the formation of oxidized forms of S-volatiles.
Addition of nitrogen may be effective in reducing further H2S and complex sulfide formation. In synthetic grape juice media with differing nitrogen content, only a few commercial strains responded to nitrogen addition by reducing the level of H2S formation. The observed impact of nitrogen addition in production conditions may be indirect and due to the stimulation of fermentation rate and not greater incorporation of reduced sulfide into amino acids. The signaling role of H2S and its potential to inhibit other microbes in the environment, offers an explanation of why formation of this compound has been selected for inSaccharomyces and why there would be diversity in the genetic path chosen to enhance sulfide production. It is possible that the nitrogen limitation leads to a deliberate release of H2S in order to inhibit other microorganisms in the environment, such that the nitrogen is available for Saccharomyces. In this case, nitrogen supplementation again has a secondary impact on sulfide release.
Hydrogen sulfide can also be removed by copper treatment and the formation of insoluble copper sulfide. Excess copper following the treatment may need to be removed. Copper can also remove methanethiol and ethanethiol. However, the disulfide form of these compounds is not precipitable by copper and over time can lead to the re-formation of the thiols. Sulfur compounds can be removed by charcoal fining but since charcoal has low specificity, many other wine components may be removed as well. There are some reports that yeast cell ghosts or spent yeast lees can remove sulfides, but these claims have not been substantiated.
TOP TIPS FOR LIMITING OFF-ODORS DURING FERMENTATION
- Choose an appropriate yeast strain: if specific lots of juice are susceptible to off-character formation, a yeast trial should be conducted to identify yeast with a reduced tendency to produce the off-note.
- Do not underfeed or overfeed the fermentation: although the exact reason this occurs is unclear, underfeeding has been shown to increase formation of H2S in many strains. Similarly, provision of high amounts of nitrogen in combination with aging on the yeast lees can lead to the appearance of sulfur volatiles. In this case, the cells have made high concentrations of the S-containing amino acids and their derivatives and these are breaking down during aging.
- Monitor aroma: aroma should be monitored at all stages of production, with particular attention paid to the appearance of off-odors that will be difficult to treat if present in the finished wine. Steps should be taken to reduce the formation of the compounds or to encourage their loss due to volatilization.
- Monitor and change production practices: if off-volatiles accompany cold soaks or yeast-lees aging, then those practices should be modified to prevent or reduce the appearance of the off-character. Cold soaks and native flora fermentations should be checked daily for the appearance of off-notes. If off-notes arise, the soak should be ended and inoculated immediately, with the same being true for native fermentations. This will limit the need for treatment later on.
- Know your aromas: make sure everyone on the crew is familiar with the aroma of off-characters so that fermentations in which they develop can be identified quickly.
