Problem fermentations can be divided into two broad categories: issues with fermentation rate progression and off-character formation. Both types of problems are sporadic and chronic, and display a dependence upon juice composition and strain variability. Both are easier to prevent than to treat. However, complete avoidance of these problems requires a sophisticated chemical analysis of juice composition that is generally beyond the scope of the typical winery. In many cases, fermentation progression appears completely normal immediately prior to the appearance of a problem. Fermentation behavior is inherently difficult to predict due to the number of potential variables. At present, a problem fermentation is only recognized once it has arisen. There are steps that can be taken to restore yeast vitality, but the success of such efforts is dependent upon correct diagnosis of the root cause of the problem
Problem Diagnosis: Fermentation Rate and Progression
Overview
There are several fermentation rate and progression issues that can arise during grape juice fermentation: long lags before onset of fermentation, a too-slow or too-rapid rate of fermentation, a sluggish maximal rate of fermentation, a slowing of fermentation, and actual cessation of sugar consumption. Careful analysis of fermentation conditions and of the fermentation profile can provide clues to the reason for poor fermentation performance. Astute monitoring of the fermentation can assist the winemaker in early identification of problem fermentations. Proper analysis of juice composition and careful attention to yeast nutritional and physiological needs can reduce the incidence of fermentation arrest. Minimizing shocks to the cells during fermentation (super heating or super cooling; high competitive bioloads) will also reduce the incidence of fermentation arrest.
The conditions of fermentation, for example, temperature, pH, aeration, level of solids, inoculation practices, can all impact the “normal” fermentation rate without leading to an incomplete fermentation. What is typical for a particular strain or fermentation condition needs to be clearly established in order to be able to confidently identify abnormal behavior. In many cases, a lack of information regarding normal expected fermentation performance seriously compounds the ability of the winemaker to quickly identify and correct problem fermentations.
Successful restarting of a stuck fermentation depends upon two critical factors: proper pre-conditioning of the yeast to be used as the inoculum and knowledge of the cause of the fermentation arrest. The latter will directly impact the former, as the tolerances of the strain used in the re-inoculation must compensate for the specific stresses of the arrested fermentation. Cells can rapidly lose viability in an arrested state, depending upon the nature of the inhibitory condition and the ethanol concentration at the time of arrest. When dealing with arrested fermentations it is important to keep in mind that the clock is ticking on culture health and vitality, and the longer the delay the higher the percentage of non-viable cells. Cell death leads to the release of components that are detected by viable cells, leading them to shut down metabolic activities rather than risk loss of viability. Fermentations can be challenging to restart, even with a fresh inoculum, if cell death has occurred.
Fermentation Progression: Typical Fermentations
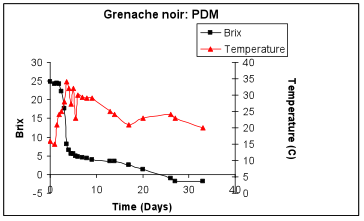
The first step in identification of the cause of a decrease in fermentation rate is a thorough understanding of the characteristics of a normal fermentation profile. The figure displays a typical Brix fermentation curve for the commercial strain Cote des Blancs in Grenache noir must harvested at 26 Brix.
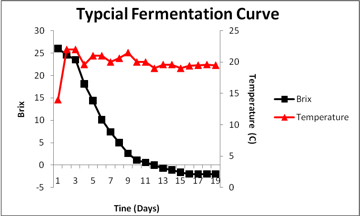
Sugar consumption initiates almost immediately upon inoculation. The highest rate of sugar utilization occurs after the cell population has reached maximal density and ethanol concentrations are too low to be inhibitory. The fermentation was complete at day 14. This is a typical profile for this commercial yeast strain.
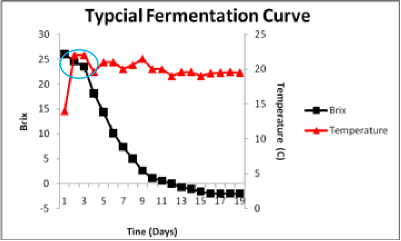
The blue circle shows the lag in initiation of fermentation. During the first 48 hours cells are adapting to the juice conditions, detoxifying juice SO2 and engaging in cell division.
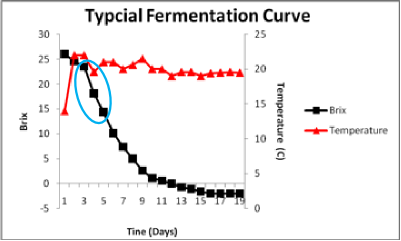
The blue circle now shows the steepest part of the sugar consumption curve. This coincides with the fastest rate of fermentation. In this particular fermentation, that high rate is sustained until the fermentation is well below 5 Brix. Given that the fermentation started at roughly 26 Brix, this indicates the strain has sustained ethanol tolerance until the external ethanol has reached approximately 11-12 % ethanol (v/v). As ethanol accumulates further, the fermentation progressively slows.
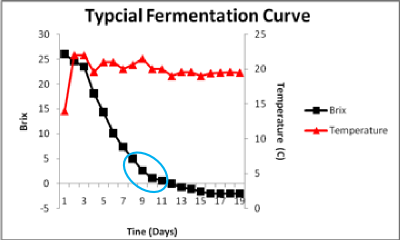
There is a distinct transition to a slower rate of fermentation. The blue circle shows the change from the initial faster rate to the new slower rate. The rate slows because ethanol in the medium forces an adaptation of the plasma membrane. The yeast can easily form a membrane that depends upon ethanol replacing water for its structure and functionality if sufficient survival factors are present to allow the new membrane to be made. Survival factors include nitrogen, sterols and fatty acids. If sufficient oxygen has been given to the fermentation early on, the cells will be able to make the necessary sterols and fatty acids. If not, then the sterols and fatty acids will need to be provided. They can be found in more complex nutrients made from yeast extracts or from yeast ghost addition (lysed yeast cells) as the fatty acids and sterols are associated with the membranes still attached to the cell walls in the yeast ghost preparations.
This slower rate is therefore the ethanol-adapted rate. At this point in the fermentation net cell growth has ceased and sugar is being consumed to provide energy to allow cells to remain resistant to ethanol. Typical fermentation curves are often characterized by two different linear phases of sugar consumption as indicated by the circles.
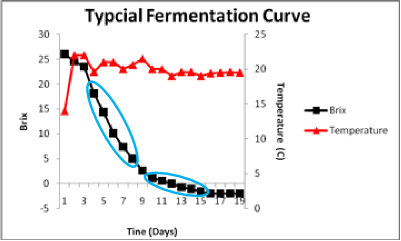
This fermentation was conducted at 20°C with good temperature control. The fermentation is warmer during the most active phase of sugar consumption because heat is released during glycolysis. Periodic fluctuation in temperature coincides with pumpovers.
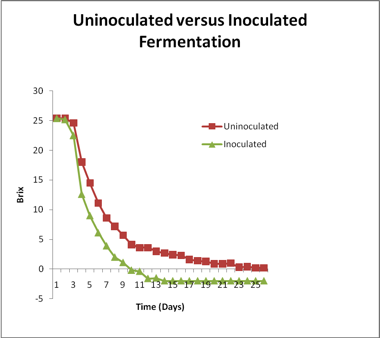
This same juice was allowed to undergo an uninocualted fermentation depicted in the following figure. The fermentation went to dryness, but the curve displays some key differences as compared to the fermentation inoculated with a commercial strain. The fermentation required an additional week for dryness to be attained. The initial rate of fermentation was more rapid than in the inoculated one suggesting that the wild strains initially present are more robust fermentors than Cote des Blancs. This fermentation displays three distinct linear phases.
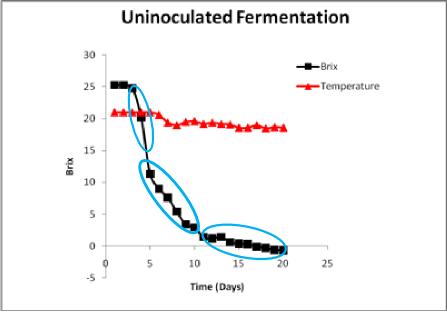
The strains rapidly initiating fermentation appear to become displaced by more ethanol-tolerant strains around 10 Brix (roughly 7-9 % ethanol). Many wild strains are not tolerant to ethanol above this concentration. The second phase is conducted by strains of greater ethanol tolerance, with the fermentation rate again showing a decline around 11-12 % ethanol. The overall rates were slower than the inoculated fermentation but the fermentation was complete. There was less temperature fluctuation, also consistent with a slower overall rate of fermentation. In some cases, rather than a rapid start, the uninoculated fermentations will show a very slow start as the population of resident Saccharomyces strains build. This is particularly true if SO2 has been used as it can inhibit the native populations. Depending upon the initial bioload level of Saccharomyces, uninoculated fermentations may lag for 7 to 10 days or longer. During this time the cells are actively dividing but many more generations are required to attain a high enough biomass level for noticeable sugar utilization to occur. Cell generation time is very much dependent upon juice nutrient content, temperature and pH. Uninoculated fermentations typically have on the order of 10 to 100 cells/mL. It will take 20 to 24 generations to reach the final cell density of 1 x 108 cells/mL compared to only 8 generations if an inoculation of 106 cells/mL is used. If the generation time is 3 hours, it will take 24 hours for the inoculated fermentation to reach final cell density but 60 to 72 hours for an uninoculated fermentation to do so.
Grape sugar is an equimolar mixture of glucose and fructose with trace amounts of sucrose, mannose and galactose. To illustrate the differences in utilization of glucose and fructose, a synthetic juice fermentation with monitoring of glucose, fructose and viable cell count is presented in the next figure.
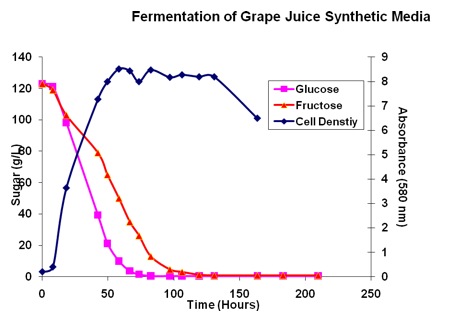
Glucose (pink line) is consumed at a faster rate than fructose (red line). In the beginning both sugars appear to be consumed at similar rates but after roughly 24 hours, the rates begin to deviate. Glucose is completely consumed by 70 hours, in this figure, while fructose is not completely consumed until roughly 120 hours. Thus, at the end of fermentation, the juice contains essentially pure fructose. The Brix curve represents the summation of the sugar values and drops below 0 because the specific gravity of an ethanol mixuter is below that of water.
The same synthetic juice fermentaiton was conducted under conditions limiting availability of fatty acids, diagrammed in the growth curves below. Unsaturated fatty acids are needed for ethanol tolerance and must be provided to the fermentation or sufficient oxygen must be available to allow their biosynthesis. In this case, cultures were anearobic, no oxygen provided, and the medium was not supplemented with fatty acids.
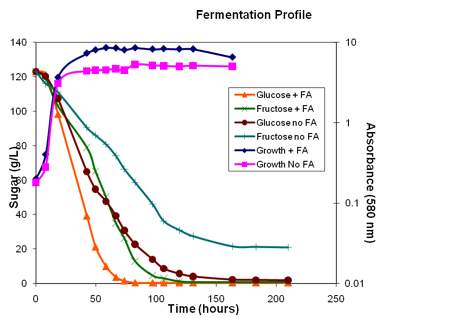
The growth curves indicate that the absence of fatty acids did not completely block growth, but the culture did not attain the same population density as the culture given fatty acids. Further, both conditions demonstrate a faster consumption of glucose than of fructose. However, in the case of the fatty-acid-limited condition, the differences in glucose versus fructose consumption rates are almost immediately apparent and a rather high concentration of fructose is left at the end of the fermentation. Oxygen/fatty acid limitation results in high residual fructose concentrations in the medium.
Fermentation profiles may differ by the yeast strain and compositional conditions. In the following figure, the fermentation displays a sustained rate and does not appear to have the distinct transistion point indicating an inhibitory concentration of ethanol has arisen in the must.
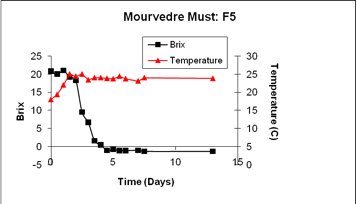
The starting Brix of this must was just slightly above 20. In this situation, dryness is attained at a low ethanol level, thus the fermentation curve does not show an ethanol-induced transition to a slower rate of fermentation.
The following fermentation shows a long lag. The fermentation is slow to initiate as the inoculum becomes dominant, but once it is dominant, the rate of fermentation is sustained over the time course of the fermentation.
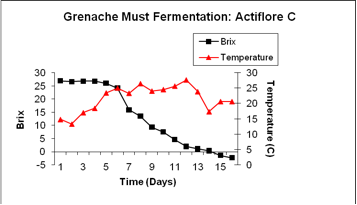
The long lag suggests that conditions were inhibitory early on, either due to high bioloads from the vineyard or use of too high a concentration of sulfite. But once the strain adapts to the conditions of the must, the fermentation proceeds to completion.
UCD522 was inoulated into the same Grenache noir must. This strain did not display a long lag but did show a more typical transition at a high ethanol concentration.
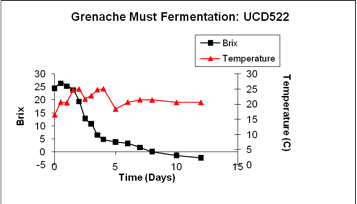
In general, an uninoculated fermentation will likely be slower than an inoculated one, but the profiles of fermentation may be quite similar depending upon the yeast inoculum used and its characteristics.
There are several components of the fermentation profile that can be evaluated in order to define what is typical for a particular strain. Length of lag, maximum fermentation rate and duration, transition point (point at which ethanol becomes inhibitory) post-transition rate of fermentation, comparison of pre- and post-transition fermentation rates and the overall time of fermentation (from lag to dryness) can all be measured easily from the graph and the information used to build a historical profile of normal for a particular strain. This type of strategy can also be used for uninoculated fermentations as well. The availability of such a database in the winery will allow more rapid determination of abnormal fermentation performance.
Types of Fermentation Progression Problems that Can Occur
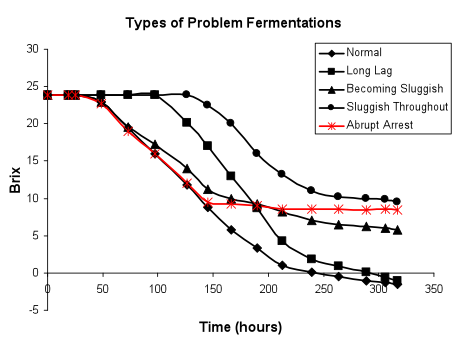
The sugar consumption pattern of problem fermentations can be a useful diagnostic tool for the winemaker. Slow fermentations can be broadly divided into four types: sluggish initiation with rate eventually becoming normal; normal initiation becoming sluggish; sluggish throughout the entire time course; and an abrupt stop. Typical types of fermentations are shown in the following figure.
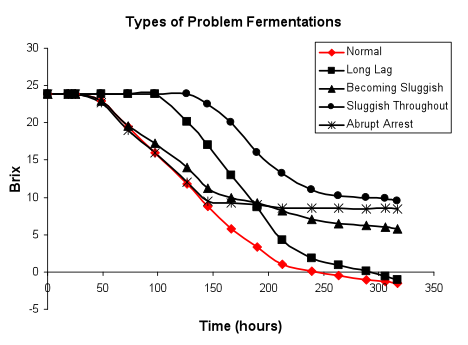
Fermentations of the first type, with a sluggish initiation, generally can go to dryness depending upon the cause of the problem and whether or not the lag in initiation generates a secondary problem such as high populations of competing non-Saccharomyces organisms. The other three types of slow fermentations may eventually go to dryness or arrest and become stuck. The different types of slow fermentation profiles are a consequence of distinct kinds of stresses imposed on the yeast and the timing of imposition of the stress.
Slow initiation of fermentation, rate becoming normal (in red below): Slow fermentation initiation generally reflects either the presence of a toxin, high viscosity, specific fermentation conditions (such as low juice temperature) or a deficient population of healthy starting yeast.
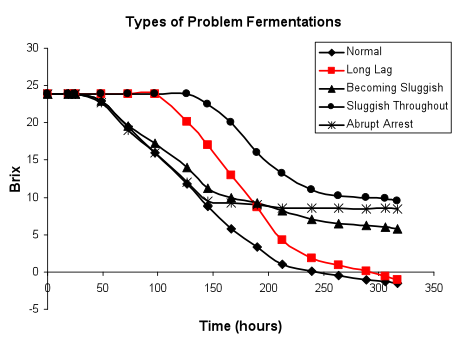
This type of fermentation profile may occur with either uninoculated or inoculated fermentations. In uninoculated fermentations, the sluggish start may simply be due to low numbers of Saccharomyces in the must, and not indicative of any particular problem other than an initial low biomass. We have found that fermentations will finish reasonably well with as little as 100-1000 viable Saccharomyces cells/mL present at the beginning, depending upon juice conditions and the relative numbers of other types of microorganisms. If it is desired that the fermentation initiate within 24-48 hours, then an inoculum of approximately 106viable cells/mL should be added. Fruit coming in from the vineyard may be deficient in Saccharomycespopulations (less than 100 viable cells/ mL), but we have found that after the first few weeks of crush, the passage of juices and musts through winery equipment has elevated the winery populations ofSaccharomyces. Cell counts of juice and must post-processing can be raised to a desirable range (104 -105cells/mL) just by transit through winery equipment, depending upon sanitation practices and the particular strains of Saccharomyces that have colonized the winery. Holding of juices and musts at low temperatures enriches for Kloeckera apicuIata (Hanseniaspora uvarum) and decreases the numbers of Saccharomycesstrains present. Saccharomyces is not as low-temperature-tolerant as other yeasts. It should be noted that addition of SO2 may not impact the viability and persistence of non-Saccharomyces yeast strains unless the amount added is over 50 mg/L. If Saccharomyces is able to eventually dominate the fermentation, these fermentations should be able to go to dryness, provided that nutrient consumption by the non-Saccharomyces yeast and bacteria has not created a deficient situation nor have toxic substances been produced. Nutrient supplementation of musts held at low temperature should be carefully evaluated. Such practices may feed the bacteria and non-Saccharomyces yeasts differentially, leading to high production of potentially toxic (to Saccharomyces) end products. The lower the initial population of Saccharomyces, the more the juice will have to provide components necessary for cell growth. For example, a juice with an initial concentration of Saccharomyces of 100 cells/mL will have to undergo 13 generations to reach a typical inoculum level of 106 cells/mL. On the other hand, fermentations starting at 106 to 107cells/mL will largely be conducted by non-proliferative cells and the nutritional requirements of such cells appear to be different than that for growth. With a high initial inoculum, deficiencies in stationary phase nutrition may be more apparent.
In the case of inoculated fermentations, poor starter culture preparation can often be a factor. Fermentations may be inoculated in two ways: either directly using rehydrated active dry yeast or from an active fermentation or starter culture. Both types of practices may be fraught with difficulty.
Use of Active Dry Yeasts:
If rehydrated active dry yeast are to be used, it is very important that manufacturer’s instructions be followed particularly with respect to both the medium and temperature used for rehydration. The first step of any winery standard operating procedure should be to check the instructions on the packet. Some yeast perform better if hydrated under the specific recommended conditions. The maximum temperatures listed for rehydration should not be exceeded as this will result in a lethal temperature shock to the yeast. Similarly, if the rehydration temperature is too low, the viability of the starter culture will also decrease. The temperature of rehydration should be between 35-40°C (95-105°F). It is likewise important to use appropriate rehydration media. Some strains tolerate rehydration in water or juice, while others only rehydrate properly in water. Use of wine for rehydration is not recommended as this frequently leads to problems with viability due to the ethanol shock to the yeast during rehydration. Other conditions of the rehydration are equally important. The yeast should be added slowly to water that is being vigorously agitated, taking care that clumps do not form. Propeller mixers are useful for this purpose. The yeast should not be allowed to sit longer than roughly 15 minutes in water before being thoroughly mixed with the must or juice. We routinely add the yeast inoculum to red must while doing a pumpover and, with white juice, we add it to bottom of the tank prior to filling from the press. If the yeast is not mixed in well, it will not be dispersed in the must, and fermentation can initiate sluggishly. Some strains perform better if rehydrated in the presence of some nutrients, particularly sugars, as this provides a source of energy for the cells. Several studies have shown that if nitrogen is limited, but sugar is plentiful, the cells will start accumulating phosphate, sulfate and other organic and micronutrients in the medium. The rehydration step can be used to feed the inoculant population preferentially. Manufacturer’s directions should always be followed.
Common Problems with Inoculations:
It is important for wineries to develop standard operating procedures for processes like rehydration and inoculation and to make sure all individuals with the responsibility for strain preparation know what procedure should be followed. Common mistakes are: adding the yeast to water that is too hot, mixing the yeast and SO2 together to save time, not bothering to rehydrate the yeast strain, using yeast packets past their pull dates and inadequate mixing of the inoculant or the tank following inoculation. If low temperature juices are inoculated, the yeast strain will have to adapt to the lower temperature. However, other wild yeasts present in the must or juice will be able to grow, putting the inoculum at a greater disadvantage. Generally, this just leads to a longer lag but does not prevent the Saccharomyces strain from dominating and completing the fermentation.
Some yeast strains are very sensitive to sulfur dioxide. We find that it is important to mix the SO2 into the tank prior to inoculation with yeast and to make sure that the SO2 is well dispersed. If it is not, then the yeast may hit a layer of a toxic level of SO2. Under no circumstances should the yeast and SO2 be mixed together in the inoculum!
Use of Fermenting Must as Inoculum:
Use of fermenting must as an inoculum can also be problematic. If the ethanol content of the fermenting must is too high at the time that it is used as an inoculum, then the yeast may be subjected to osmotic shock upon addition to fresh must. Cells that have already adapted membranes and protein content to high ethanol conditions will have to “de-adapt” upon abrupt dilution of the inhibiting ethanol in fresh medium. We have found that the best results are obtained if the ethanol content of the starter is around 3-5%, but no higher than 7% (v/v).
Further, use of fermenting must as inocula may result in use of nutrient-depleted cultures. In general, for a vitamin deficiency to become manifest in a culture of healthily growing yeast, roughly 40 generations of growth in the absence of the vitamin is required. If active dry yeast is used as inoculum, micronutrient-deficient juices will not lead to a starvation situation for the yeast since these cultures have been enriched in micronutrients. However, if the inoculum is serially passaged through juices, micronutrient deficiencies can arise that will inhibit not only the initiation of fermentation but its progression as well. We have found that a starter can be passaged once to fresh juice without loss of fermentation initiation ability. With a second passage, some fermentation problems may arise, but a third passage of the inoculum can lead to a sluggish if not stuck fermentation.
Inoculation Management Techniques:
Sluggish initiation of fermentation may also be caused by poor strain management techniques. If there is a dramatic difference between the temperature of the inoculum and that of the must, the yeast will receive a temperature shock which may impact continued viability. This is frequently a problem with juices that have been cold-settled, then inoculated prior to reaching a temperature that is warm enough. In such cases, it may be better to start the yeast in a fraction of pre-warmed juice, and once the yeast have started, use this mixture as the inoculum of a low temperature fermentation.
Over-clarified juices may also initiate slowly due to a low solids content. A current fashion in the California wine industry is to reduce the solids content of the white juice so that the finished wines will not need much, if any, fining and filtration. Wineries using this approach need to determine if such practices are associated with a higher incidence of stuck fermentations.
Similarly, heat treatments of musts and juices can lead to a loss of nutrients and over-settling and clarification. If such treatments are used on marginal fruit, particularly in cases of moldy clusters, the mold and other accompanying organisms may not be completely inactivated by the heat treatment. Their numbers may be reduced but not eliminated entirely. The bacteria, in particular, can rebound after such heat treatments, so addition of sulfite may be necessary. If nutrients have been lost in the heating process, nutrient additions can be made to compensate.
Slow initiation of fermentation, fermentation sluggish throughout (in red below): Problem fermentations displaying a sluggish initiation may not recover and may remain slow over the entire time course of sugar consumption. Such fermentations generally reflect a problem with attainment of maximal viable biomass. The causes of this type of problem are numerous and can indicate a problem in cell growth, maintenance of viability or both.
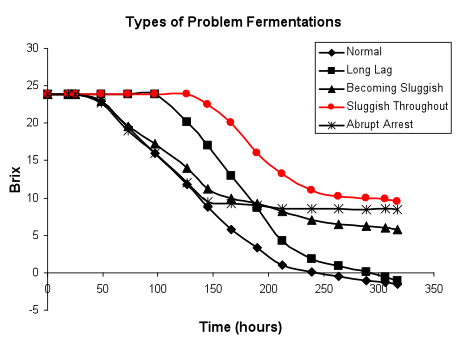
Saccharomyces cell counts can easily be used to determine precisely if a culture is unhealthy. Under normal conditions, at approximately 48-72 hours into the fermentation at 18 - 25°C, suspended cell counts should be around 107 to 108 cells/ mL, with a viability of 80 to 100%, depending upon the wine strain used and the conditions of inoculation and fermentation. If the cell counts or percent viability are significantly below these values, and there is no extenuating circumstance such as heavy use of SO2 or a very low temperature of fermentation (12-14°C or below), then the fermentation will likely be at high risk for being sluggish throughout and may even arrest. The cell count may fluctuate during fermentation by as much as a factor of 100 (106 to 108) as subpopulations of yeast settle and new growth occurs, but the percent viability should remain high. When determining cell counts, it is important to only evaluate the suspended population; the settled populations should not be mixed and resuspended. It is also important to distinguish betweenSaccharomyces cells and those of other organisms, especially the non-Saccharomyces yeasts, in the fermentation.
Problems in attainment of maximal biomass typically indicate a nutrient deficiency or suboptimal growth conditions, such as a low pH. Difficulty in maintenance of maximal viable biomass can indicate a severe deficiency of survival factors, increasing ethanol or acetaldehyde sensitivity, presence of zymocidal (toxic to yeast) substances, or poor strain tolerances to stress.
Normal initiation of fermentation, rate becoming sluggish (in red below): Frequently fermentations initiate normally, attaining both maximal biomass and fermentation rate, but fail to maintain hexose consumption, gradually slowing and becoming sluggish.
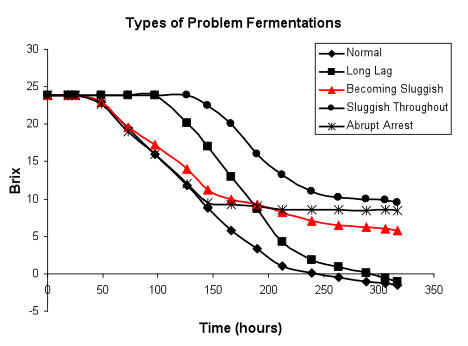
This type of fermentation profile suggests that nutrients required for growth were plentiful and conditions fully permissive for proliferation. These types of problematic fermentations appear to be due to difficulty in maintaining metabolic activity or viability during the non-proliferative or stationary phase. This is the most common type of arrest seen – a normal initiation with no indication of an ensuing problem. A moderate deficiency in survival factors can lead to problems in ability to tolerate ethanol or acetaldehyde that will not be apparent until ethanol accumulates in the medium. More severe deficiencies may impact growth as well. The role of fatty acids and sterols in ethanol tolerance is well known and preparations containing these components can be used as nutrient additions. However, it is important to add these compounds to the must prior to the arrest of fermentation or it may be too late to rectify the problem. The current tendency to harvest fruit at high initial sugar content may result in inhibitory ethanol concentrations, and be a contributing factor to the appearance of this type of slow fermentation. This may be alleviated by blending such musts and juices with those of a lower initial Brix. The presence of zymostatic or zymocidal toxins that are more toxic at higher ethanol levels can also be a cause of a late arrest of fermentation. An imbalance of potassium and hydrogen ions yields such a fermentation profile, suggesting that problems in regulation of hydrogen ion fluxes into the cell can seriously impact non-proliferative phase metabolism. We have also seen very high inoculum levels of yeast (108 cells/mL) result in a sluggish fermentation. These fermentations initiate very rapidly, but seem to progress slower than fermentations that build a population of yeast rather than starting at maximal cell density. These fermentations display greatly elevated levels of yeast esters.
One area that merits further investigation is examination of the factors important in stabilizing soluble cytoplasmic functions in the presence of high ethanol. Ethanol can disrupt enzyme activity and organelle function as well as perturb the cell’s permeability barrier. Recent work suggests that trehalose, proline and glycine are all important factors in stabilizing internal components. The first two, or their precursors (glucose for trehalose; arginine for proline), are generally plentiful in grape must. Glycine is not. These compounds do not appear to be able to substitute for each other in very high gravity fermentations. Nitrogen deficiency may impact the yeasts' ability to synthesize protective factors and lead to enhanced ethanol sensitivity of cytoplasmic functions. Further, since ethanol induces leakage of several compounds from the cell, higher levels of these components than are required for growth may be necessary. The impact of acetaldehyde accumulation on fermentation arrest also needs to be further studied. Factors leading to a reduction in alcohol dehydrogenase activity may increase cytoplasmic acetaldehyde to toxic levels.
A final cause of this type of fermentation arrest may be a mild temperature shock. Sensitivity to temperature increases at high ethanol concentrations, and, if a fermentation is being cooled, the temperature may drop below permissive levels, depending upon the type of refrigeration being used and its control. It is also important to remember that most cooling jackets do not provide for a uniform temperature across the tank. The area immediately adjacent to the cooling system (jacket or insert) will likely be much cooler than the rest of the tank.
This graph shows an example of a fermentation that became too hot during the initial stages of sugar consumption. There is a too-rapid initiation of fermentation and a dramatic slow down of sugar utilization around 8 Brix. The ethanol content at this time is around 9%. Depending upon the strain, early heat shocks do not become manifest as a fermentation problem until ethanol accumulates to 8-11%, depending upon the strain. In this case, it appears that an ethanol tolerant subpopulation was able to eventually grow and complete the fermentation, but this is not always the case. The dramatic swings in temperature in this fermentation are due to the pumpover regimen and mixing of the tank, with the concurrent dissipation of heat during this process. Such hot starts of fermentations generally lead to reduced complexity of the wine.
Premature settling of the yeast population is also a good indicator of a problem in maintenance of metabolic activity during stationary phase. We have observed an early settling in fermenting musts held too long at a low temperature (below 20°C) following the cessation of active fermentation. In many cases, readjustment of the temperature does not result in an improved fermentation rate.
Fermentation normal, abrupt stop (in red below): An abrupt cessation of sugar consumption is usually indicative of a major traumatic shock to the fermenting yeast. This might be due to exposure to extreme temperatures. Uncooled fermentations may attain an inhibitory temperature due to the release of heat during hexose catabolism, as shown above. Similarly, fermentations can be overcooled depending upon the design of the system being used.
Other types of shocks, such as a mistake in addition of sulfur dioxide, can also lead to an abrupt stop of the fermentation. In one case, filtration of an incomplete fermentation through a pad filter contaminated withStreptomyces, led to an abrupt arrest of fermentation. Similarly, addition of a malolactic starter culture to a fermentation that is not yet dry can lead to a rather abrupt arrest, depending upon the biological activity, size of the inoculum, nutritional complement of the must, presence and nature of organic and fatty acids in the ML inoculum and the particular yeast and bacterial strains involved. Depending upon the pH, organic acids released by bacterial metabolism may be protonated and, therefore, simply diffuse across the yeast plasma membrane. Once inside the cytoplasm, the acids release protons due to the higher internal pH. Yeast can use the plasma membrane ATPase to pump out the hydrogen ions coming from the acids. However, if the capacity of the pump is saturated by pumping out the hydrogen ions coming in from the enhanced passive proton flux due to ethanol, then the yeast will not be able to tolerate the acid addition and fermentation will rapidly arrest. Bacterial fatty acids can become inserted into the ethanol-adapted plasma membrane, disrupting sugar uptake and leading to cell death. Some yeast strains tolerate ML bacteria additions much more readily than others and some ML strains are less prone to cause a problem.
Premature addition of fining agents can also lead to loss of culture biomass and dramatically slow the fermentation. These factors are all generally well within the control of the winemaker and should not routinely pose a problem.
Restarting Arrested Fermentations:
Restarting an arrested fermentation can be challenging, depending upon the cause of the arrest. If fermentation is inhibited due to a nutrient deficiency of either a macronutrient like nitrogen, a micronutrient like biotin, or a mineral ion, addition of the missing component is often enough to restart fermentation. Yeast frequently are easily able to recover from a cold shock if the tank is warmed and mixed to resuspend the population. It is more difficult to restart fermentations arrested due to ethanol intolerance, heat shock or presence of inhibitory organic and fatty acids. In the case of heat shock, survival of the elevated temperature forces changes in plasma membrane composition incompatible with tolerance of the membrane to ethanol. Thus, the alcohol tolerance level is reduced and cells will arrest at a lower than normal ethanol percent.
High ethanol can cause a phase change in the membrane that the cells cannot easily repair. Reducing the ethanol content via dilution likely will not restore the functionality of the membrane. The membrane can only be restored if cells are returned to fully permissive growth conditions so that a new membrane can be made for the daughter cells, which is difficult to do for a fermentation already above 10% ethanol.
If inhibitory acids are present the acids level will need to be reduced before growth can commence. If the acidity has lead to acidification of the cell cytoplasm the cells will not recover. A new inoculant will be just as susceptible to inhibition by the existing acids. Sometimes use of yeast ghosts or inactivated yeast cells to sop up the fatty acids or other inhibitors present in the medium followed by removal of the yeast biomass and reinoculation can help restart fermentations arrested due to the presence of inhibitors.
Regardless of the cause of the fermentation problem, it is frequently necessary to first rack the wine off of the settled yeast lees before attempting to restart the fermentation. Some types of arrested fermentations may restart without addition of yeast following this treatment. We have found from analysis of juice-like media wherein the cause of fermentation arrest can be strictly controlled, even moderate aeration will lead to a spontaneous restart of fermentations arrested due to sterol or fatty acid limitation, provided that is the only limitation and the restart is conducted in a timely fashion. Nitrogen or micronutrient limitation on top of either an oxygen deficiency or temperature shock, will inhibit a spontaneous restart.
Another problem with reinitiating stuck fermentations concerns the timing at which intervention will have a positive outcome. It has been well established that nitrogen limitation must be corrected before a cessation in fermentation occurs. We have seen the same effect of potassium addition. By the time a fermentation has arrested due to an imbalance of potassium and hydrogen ion concentrations, it is too late to correct the problem by adjustment of the ratio of the two ions. If the stuck or sluggish fermentation is an adaptive response to adverse conditions, the readjustment of the medium must occur prior to commitment to that adaptation. A decrease in fermentation rate is frequently a consequence of adaptation and not a cause. Thus, it is desirable to develop diagnostic tools for the early identification of a problem fermentation, preferably prior to significant loss of transporter activity. It would also be useful to develop better means by which to determine the precise cause of the stress so that it may be rectified. Frequently the circumstances preceding the arrest of fermentation and the type of change of the fermentation profile can provide key information as to the likely cause of the problem.
Restarting Procedures:
There are three different types of strategies for restarting arrested fermentations. It is helpful if some idea of the cause of the arrest is known, but if not, there are options that can be used to complete the fermentation:
- Rejuvenating the existing biomass
- Reinoculation with a new adapted inoculum
- Use of activated encapsulated or yeast-in-bag processes
Rejuvenating Existing Biomass
If fermentation has ceased due to a reversible inhibition of the culture, then rejuvenating the biomass can lead to restoration and completion of fermentation. Reversible inhibition would be a low temperature shock, a mild deficiency of survival factors or a modest nutrient limitation. Raising the temperature, aerating the biomass and provision of nitrogen and cofactors can restore fermentation rates. Often, it is not known if a fermentation will restart from the existing biomass. The ability to restart from the existing biomass can often be determined by some quick bench trials. Samples of the tank can be taken and subjected to different treatments isolation and in combination, nutrient addition, aeration, temperature increase, to see if fermentation reinitiates. The successful treatment can then be applied to the entire tank. It is important that the bench trials be conducted under conditions that mimic what will happen in the larger production tank. There may be better mixing in the bench trial that will not be replicated in the larger tank.
If a microscope is available, it is also advantageous to examine the arrested population under the microscope and compare it to a healthy population from another tank. If the yeast from the arrested population appear lysed (popped open), or have granules inside that are moving by Brownian motion, then the population is in decline and will be much harder if not impossible to rejuvenate.
Before attempting rejuvenation, it is important to first check the tolerances of the strains and compare them to the fermentation conditions. A frequent cause of arrest of fermentation is use of a yeast strain that does not have the ethanol-tolerance level needed to complete a fermentation. The ethanol-tolerance level of commercial yeast strains is generally known. A good rule of thumb is to assume a worst-case scenario with respect to Brix yield of ethanol, 0.62 x Brix value, and be sure the strain has the ethanol tolerance capacity to attain this level. Fermentation of a 24 Brix juice would require an ethanol tolerance of 15%. 28 Brix would require a tolerance of 17%. Tolerance to ethanol is impacted by growth conditions, so the tolerances listed by manufacturers are not absolutes, but do provide a good estimate of the level of ethanol at which the strain can be expected to arrest. Strains also differ in tolerance of temperature shocks, nutrient limitation and bacterial competition. This information is also generally known for commercial strains. Before considering a rejuvenation strategy an assessment of the inoculant strain should be undertaken. If it does not have the tolerances needed to complete the fermentation then a reinoculation strategy with a more tolerant strain should be employed.
Reinoculation with a New Adapted Inoculum
If the fermentation arrest is due to ethanol intolerance, to temperature or acid shock, or to poor innate tolerances of the strain dominating the fermentation, it will be necessary to reinoculate. The new inoculants will have to be adapted to the conditions of the arrest. If the ethanol concentration is above 7% the new strain may have difficulty adapting to the fermentation conditions and will have to be introduced to the ethanol concentration in a gradual fashion. For example, mixing half of the arrested wine with fresh juice to drop the ethanol concentration to or below 5% will assure a healthy start for the new inoculant. If enough fresh juice is not available, water and commercial nutrients can be used to dilute both the juice and wine mixture to a permissive alcohol level. Once active fermentation is evident, meaning that obvious fermentative release of carbon dioxide is occurring, then more and more of the arrested ferment can be added in stepwise fashion taking care to not let the ferment go dry at any time in the process.
Several commercial strains are available that have been isolated specifically because of their low nutrient requirements, high ethanol, temperature and bacterial metabolite tolerances, and ease of rehydration. One of these strains should be considered instead of using the initial strain. The availability of a microscope greatly enhances the ability to monitor the health and vitality of the new inoculant. It is also important to not add excessive sulfite when attempting a restart or to add the ML bacteria at the same time as the attempted restart.
Use of Activated Encapsulated or Yeast-in-Bag Processes
Growing a new inoculant, taking care to make sure it is adapted to the conditions of the arrested fermentation, can often be a time consuming process with no guarantee of success. It is often easy to let the new culture go too far, that is, it consumes all the available sugar in one mixture and arrests itself before a transfer can occur. If all that is needed is to consume the rest of the sugar and complete a fermentation, then use of an encapsulated yeast may be a better approach. Encapsulated yeast are “trapped” in an alginate matrix. The alginate beads are fully permeable to substrates and endproducts but do not allow growth of the yeast cells. The reinforcement of the alginate matrix puts the yeast in a biofilm like mode of metabolism. Under these conditions substrates can be consumed efficiently and cells are not sensitive to factors inhibiting growth or disrupting membranes. The encapsulated yeast have been adapted to high ethanol and low nutrients during the encapsulation process and will work well in completing sugar consumption of an arrested fermentation, provided that other inhibitory conditions such as high SO2, high acetic acid, low pH, very high ethanol level, and/or high bacterial bioloads, do not exist or have not been rectified. The encapsulated yeast can be placed inside of a mesh bag for optimal distribution within the tank and for ease of removal. Again, if using one of these products, the manufacturer’s recommendations should be carefully followed. Extremes of temperature should be avoided as well as any other conditions that may adversely impact the viability of the yeast within the capsules.
